pH meter 14H

pH meter 14H
€57,20
14H electronic pH meter for pH measurement
Definition: 0.1 pH
Accuracy: +/- 0.1 pH
Calibration: 1 reference point,
Auto. temp. comp.: No,
Batteries: 3 x 1.5 V LR44 – not included
Environment: 0 – 50°C, RH 100%.
Dimensions (weight): 153 x 24 mm (45g)
Reference solution: 7.01 pH (20 ml sachet included)
Measurement parameters:
pH value (pH): 0.0 – 14.0
pH meter 14H
It measures the hydrogen potential (or pH) of water*. This easy-to-use, reliable device is ideal for measuring the pH of water. It immediately displays the pH value of the water. It will enable you to check the ideal value of your water and modify your treatment settings if necessary.
the pH meter is waterproof. And so it can be easily used for all measurements immediately after being calibrated with pH7 buffer solution.
It comes with an elegant leatherette case with belt clip and pH7 buffer solution for calibration of the pH meter 14H.
The pH7 buffer solution is available separately.
The reading head should be replaced after wear.
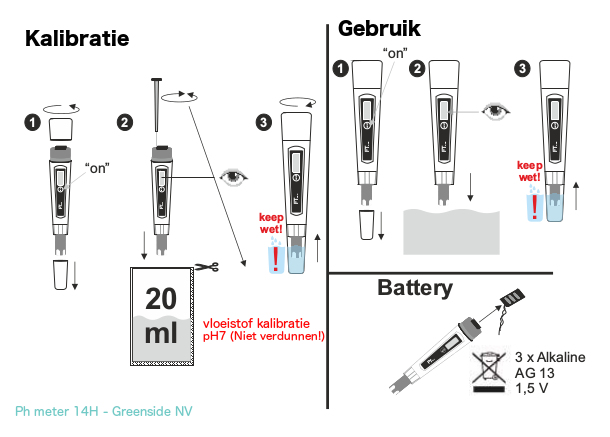
See the graphical instructions for the pH meter 14H Instructions for use
pH indicator maintenance
The pH indicator is a precision instrument. Correct use and maintenance are important factors in getting the longest possible life from your pH indicator. Here are several important points:
- The electrode is delivered dry. Soak for 2 hours in the preservative solution (or water). The protective cap on the electrode can be filled with a preservative solution (or water). This will ensure that your electrode remains operational for a much longer period of time (shorter with water).
- Before measuring, the pH indicator must be calibrated. Buffer solutions are used for this purpose. If your pH indicator can only be calibrated at a single point, you should use the buffer solution that is closest to your normal measurements (for drinking water PH7).
- Do not introduce "contaminations" into the buffer solution, and do not dilute it with water. So never insert the wet electrode directly into the claibration bottle. Use a soft, clean cloth to gently dry your electrode.
- Never touch the electrode if you can avoid it, certainly not with your fingers. If you need to clean the electrode, use only a soft, non-scouring cloth.
* pH measures the acidity or basicity of a solution. Thus, in an aqueous medium at 25°C :
- A solution of pH = 7 is said to be neutral;
- A solution with a pH of < 6.5 is said to be acidic;
- A solution with a pH of > 7.4 is said to be basic;
Cheers:
It's worth noting that if you want to compensate for the acids produced by the body in the blood and tissues, it's with water rich in bicarbonates that you can do so! The Hydrokube helps you transform limescale into bicarbonate.
It's the level of bicarbonate in the water that's important for neutralizing acids, and not so much the use of alkaline water (high pH). >7,4). By way of illustration, water such as Vichy St-Yorre is rich in bicarbonates, but its pH is acidic (below 7 and equal to 6.6)! Vichy water is said to be alkalinizing because, even though it is acidic, it is rich in bicarbonate content. Vichy water has already demonstrated its 'alkalinizing' action, for example in its therapeutic action on athletes who need to neutralize excess lactic acid.